How To Draw Ionic Bonds
How To Draw Ionic Bonds - Sodium (2,8,1) has 1 electron more than a stable noble gas structure (2,8). Web ionic bonding in sodium chloride. If it gave away that electron it would become more stable. In any molecule or ion with the general formula abn , the unique atom (a) is in the center and all of the b atoms are attached to a. 1.7.1 formation of covalent bonds; Web this crash course chemistry video tutorial explains the main concepts between ionic bonds found in ionic compounds and polar & nonpolar covalent bonding foun. Web ionic bonding the atoms form ions by gaining or losing electrons to obtain electron configurations with a full outer shell. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. In electron transfer, the number of electrons lost must equal the number of electrons gained. Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Web ionic bonding the atoms form ions by gaining or losing electrons to obtain electron configurations with a full outer shell. Web this chemistry video explains how to draw the lewis structures of ionic compounds. Web i want to help you achieve the grades you (and i) know you are capable of; Web draw a lewis electron dot diagram for. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web this chemistry video explains how to draw the lewis structures of ionic compounds. Web ionic bonding mats this resource accompanies the poster how to draw ionic bonds from education in chemistry which can be viewed at rsc.li/3amzz9j learning objectives 1 draw dot and cross. Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. It is assumed that only electrons in the valence shell are involved in the formation of covalent bonds. Web for exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for. 1.21b explain how to draw ionic bonding.more. Web this crash course chemistry video tutorial explains the main concepts between ionic bonds found in ionic compounds and polar & nonpolar covalent bonding foun. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Draw a square bracket around each ion. Web the attraction of oppositely charged. The two electrons in the bond are simultaneously attracted to both nuclei. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. Web it's just for ionic compounds electrons aren't shared so you won't have things like single bonds between atoms. Ionic bonds require at least one electron donor and one electron. Web i want to help you achieve the grades you (and i) know you are capable of; It is assumed that only electrons in the valence shell are involved in the formation of covalent bonds. An ionic compound is made up of charged particles, called ions. In electron transfer, the number of electrons lost must equal the number of electrons. Examples include nacl, mgf2, k2o, and al2o3. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Web this chemistry video explains how to draw the lewis structures of ionic compounds. Web for exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw. Web representing a covalent bond using lewis structures. 1.7.1 formation of covalent bonds; If it could gain an electron from somewhere it too would become more stable. Ionic bonds require at least one electron donor and one electron acceptor. Chlorine (2,8,7) has 1 electron short of a stable noble gas structure (2,8,8). Web when drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular compounds. Examples include nacl, mgf2, k2o, and al2o3. Web it's just for ionic compounds electrons aren't shared so you won't have things like single bonds between atoms. In almost all cases, chemical bonds are formed by. Web when drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular compounds. If it gave away that electron it would become more stable. Web for exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross. Draw a square bracket around each ion. Draw a square bracket around each ion. It is assumed that only electrons in the valence shell are involved in the formation of covalent bonds. If it could gain an electron from somewhere it too would become more stable. Web it's just for ionic compounds electrons aren't shared so you won't have things like single bonds between atoms. The two electrons in the bond are simultaneously attracted to both nuclei. Examples include nacl, mgf2, k2o, and al2o3. Web ionic bonding in sodium chloride. Web for exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. Web draw a lewis electron dot diagram for an atom or a monatomic ion. 2 show how electrons are transferred in ionic bonding. This is because valence electrons. An ionic compound is made up of charged particles, called ions. Web in ionic bonding, atoms transfer electrons to each other. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. Web representing a covalent bond using lewis structures.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
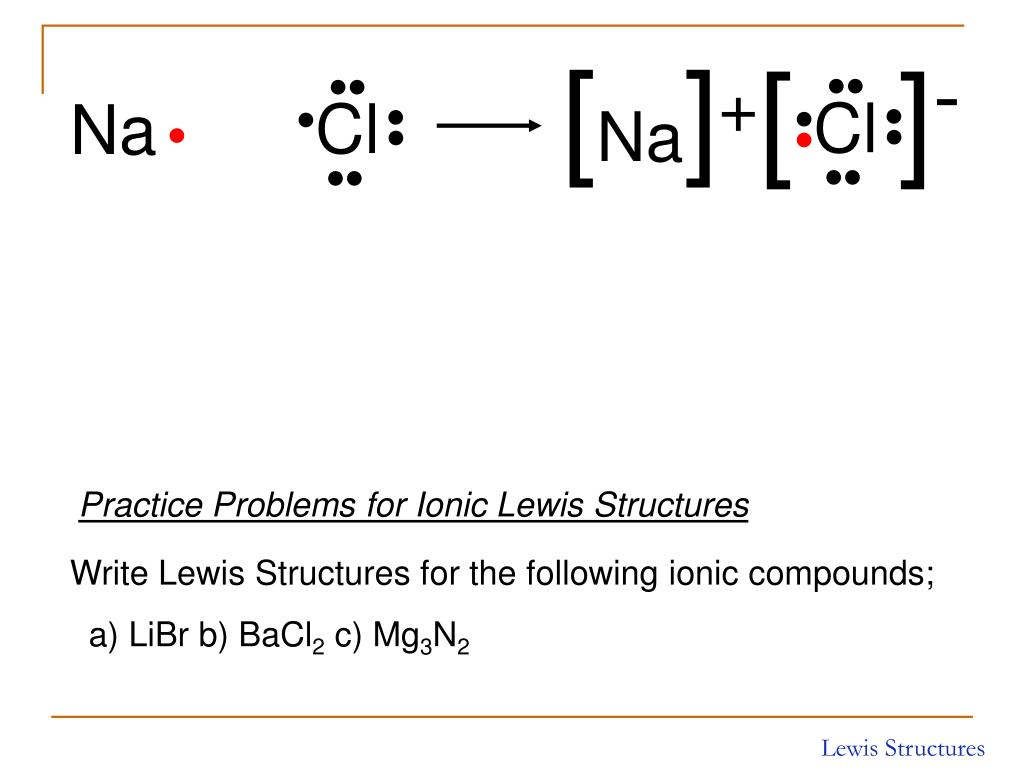
Lewis Structure Of Ionic Compounds
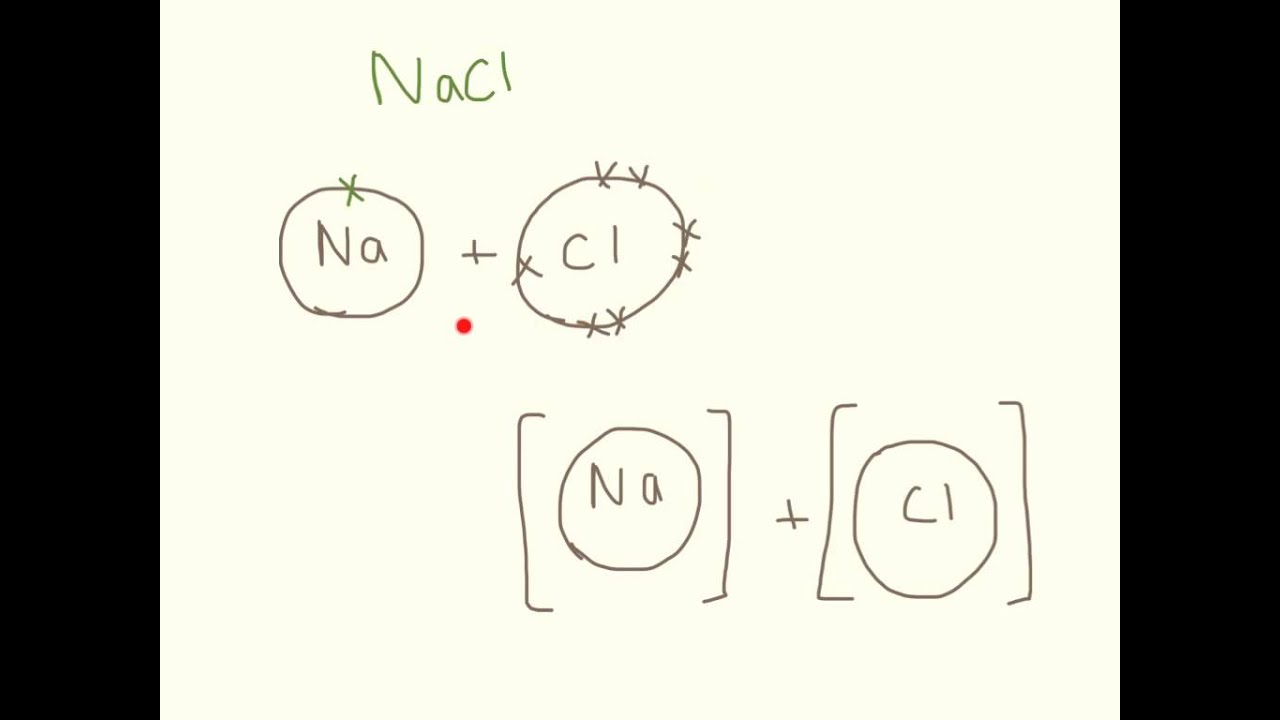
drawing ionic bonds worksheet lineartdrawingsheartflowers

How Ions Are Formed? Infrared for Health
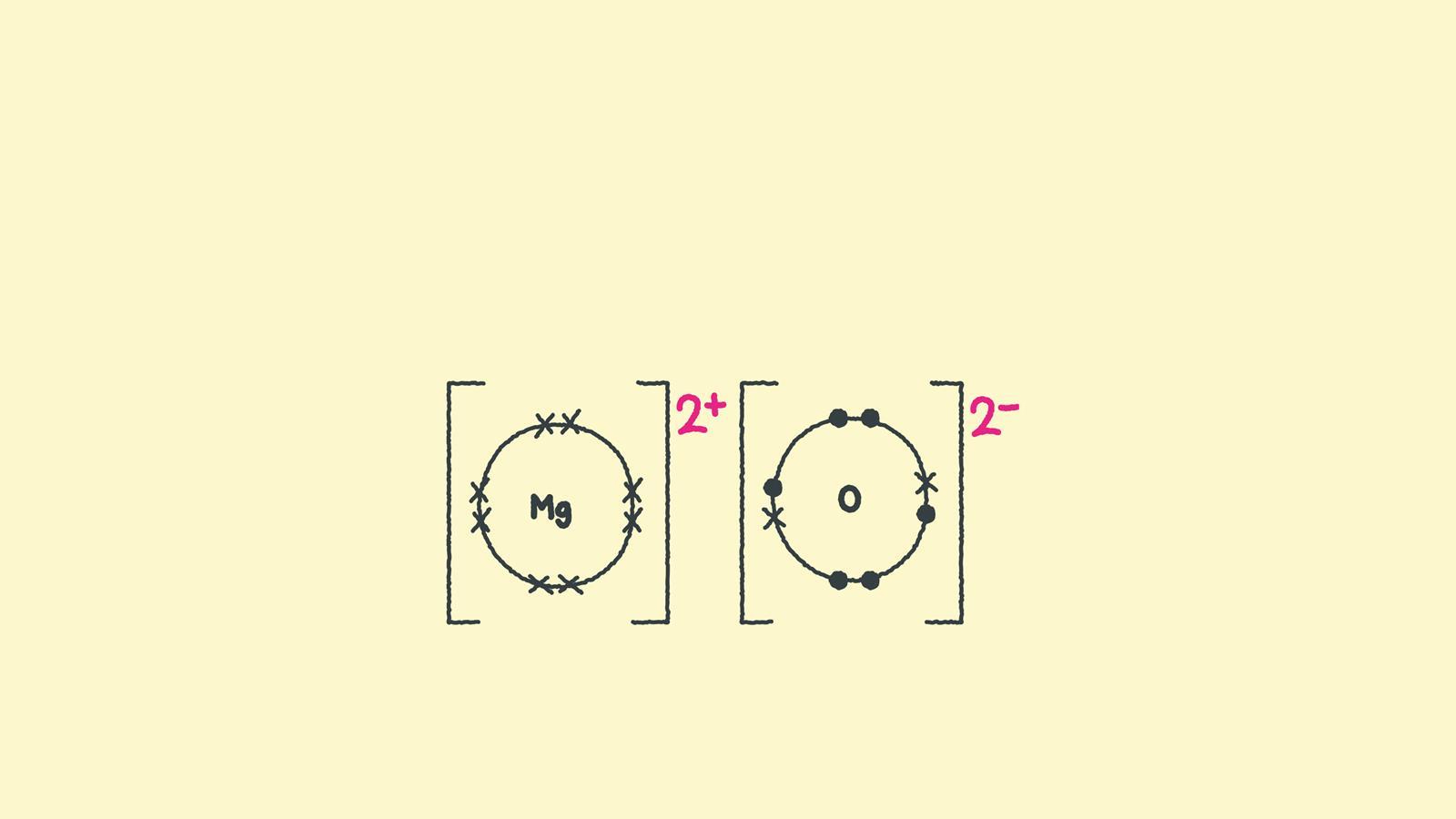
How to draw ionic bonding dot and cross diagrams Feature RSC Education

2016 topic 4.1 bonding ionic

Ionic Bonding Diagram

How Do Ions Increase Conductivity? Atlas Scientific
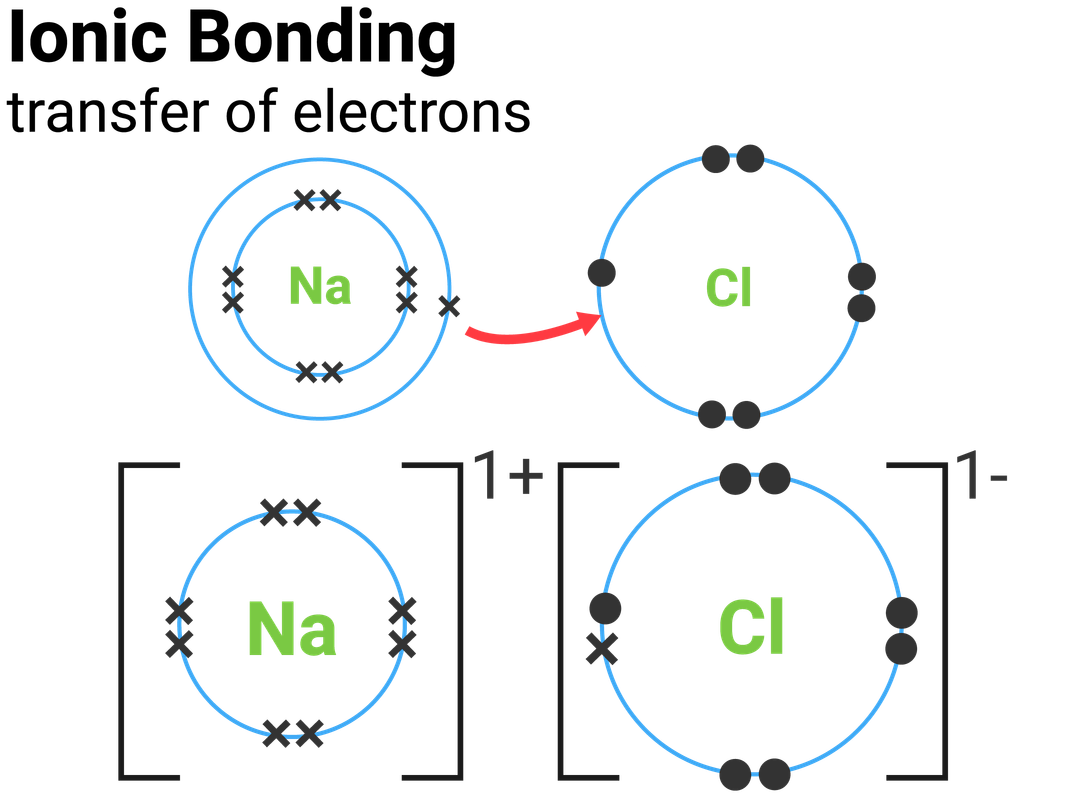
6 ionic bonding 01 — Postimages

subatomic particles Montessori Muddle
Web 6.7K Views 7 Years Ago Edexcel Chemistry 2022:
Magnesium Loses Two Electrons And Oxygen Gains Two Electrons.
Even If You Don't Want To Stud.
Web The Attraction Of Oppositely Charged Ions Caused By Electron Transfer Is Called An Ionic Bond.
Related Post: