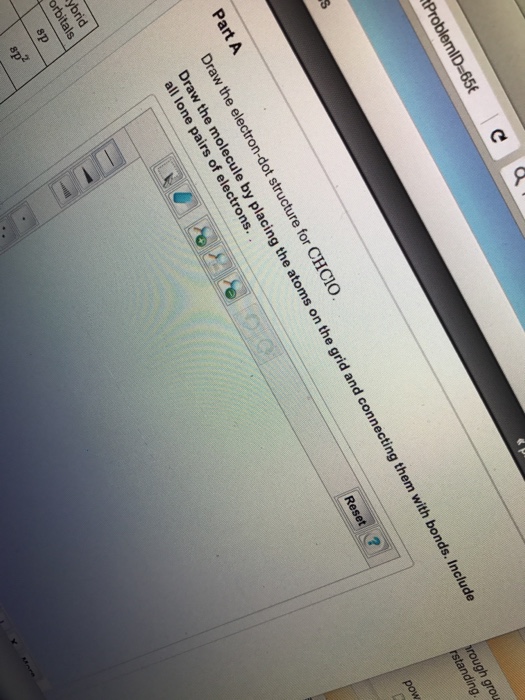
Draw The Lewis Structure For Chclo
Draw The Lewis Structure For Chclo - Understand the proper use of the octet rule to predict bonding in simple molecules. Determine the total number of valence electrons: Formyl chloride has 3 regions of electron density around the carbon, and a formal pπ −pπ c − o bond. Include all lone pairs of electrons. Web draw a lewis structure for (ch_3ch_2)_2chch (ch_3)_2. Are all the peripheral atoms in equivalent environments? The following procedure can be used to draw lewis structure for simple molecules. Web steps #1 first draw a rough sketch. Draw the molecule by placing atoms on the canvas and connecting them with bonds. U.−3 ρ b c p ( o − h) = 0.34 e a. Is brf5 a polar molecule? It is connected to hydrogen and chlorine through single bonds and to oxygen through a double bond. Web write lewis symbols for neutral atoms and ions. 01:14 draw a lewis structure for the c2−2 c 2 2 − ion. Web draw the lewis structure for chclo this problem has been solved! This widget gets the lewis structure of chemical compounds. Draw the molecule by placing atoms on the canvas and connecting them with bonds. There was an issue loading this video view best match solution instead instant text answer step 1/6 1. Web what we see here is that the bond between the hydrogen and the oxygen is strongly covalent as. Web draw the lewis structure and determine the electron domains, lone pairs, atomic arrangement, ideal bond angles, and actual bond angles of the central atom for brf5. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web draw a lewis structure for (ch_3ch_2)_2chch (ch_3)_2. U.−3 ρ b c p ( o − h) =. Count the valence electrons of each atom in the molecule. #3 calculate and mark formal charges on the. The following procedure can be used to draw lewis structure for simple molecules. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Electrons are shown as dots or for bonding electrons as a line between. Web 01:31 draw the lewis structure of h2co. #3 calculate and mark formal charges on the. Web draw a lewis structure for (ch_3ch_2)_2chch (ch_3)_2. − 3 and the laplacian has a high negative value, ∇2ρbcp(o−h) = −2.02 ∇ 2 ρ b c p ( o − h) = − 2.02, i.e. Is brf5 a polar molecule? Find more chemistry widgets in wolfram|alpha. Draw lewis structures depicting the bonding in simple molecules. Web drawing the lewis structure of formyl chloride (chclo) involves several steps. In the periodic table, carbon lies in group 14, hydrogen lies in group 1, chlorine lies in. It's the same as ch2o. Hello students in this question we have been asked to draw louis structure of c h, c l, o so, first of. Web steps #1 first draw a rough sketch. 01:14 draw a lewis structure for the c2−2 c 2 2 − ion. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web step. It is used to show how the electrons are arranged around individual atoms in a molecule. Here, we have a total of 9 electron pairs. The final answer must have this number of electrons‼! #3 calculate and mark formal charges on the. Step 3) add electrons to all outer atoms. Web jun 1, 2017 given o = c(cl)h it is trigonal planar to a first approx. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”). U.−3 ρ b c p ( o − h) = 0.34 e a. It is connected to hydrogen and chlorine through single bonds and to oxygen through a double. View more transcript video answer: Web 01:31 draw the lewis structure of h2co. Web steps #1 first draw a rough sketch. #3 calculate and mark formal charges on the. Draw the lewis structure for xef2 and determine its electron and molecular geometries. Draw lewis structures depicting the bonding in simple molecules. Draw the lewis structure for xef2 and determine its electron and molecular geometries. Draw the molecule by placing atoms on the canvas and connecting them with bonds. Web draw a lewis structure for (ch_3ch_2)_2chch (ch_3)_2. ∠cl −c −o and ∠h − c − o are roughly 120 ∘. Web steps #1 first draw a rough sketch. Draw lewis structures for covalent compounds. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”). In the periodic table, carbon lies in group 14, hydrogen lies in group 1, chlorine lies in. Step 2 2 of 3 in chclo, we have carbon as a central atom. Count the valence electrons of each atom in the molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw the lewis structure and determine the electron domains, lone pairs, atomic arrangement, ideal bond angles, and actual bond angles of the central atom for brf5. Electrons are shown as dots or for bonding electrons as a line between the two atoms.
How To Draw Lewis Structures A Step By Step Tutorial

Chclo Lewis Structure What The Molecular Shape Of Chclo Clutch Prep

Draw The Lewis Structure For Chclo
Draw The Electrondot Structure For Chclo. Carbon Is The Central Atom

Chclo Lewis Structure What The Molecular Shape Of Chclo Clutch Prep

Determine whether CHClO is an ionic or molecular compound and draw an

Hclo3

Estructura De Puntos De Lewis De Chclo Qaseem
Solved Draw the electrondot structure for CHClO. Draw the
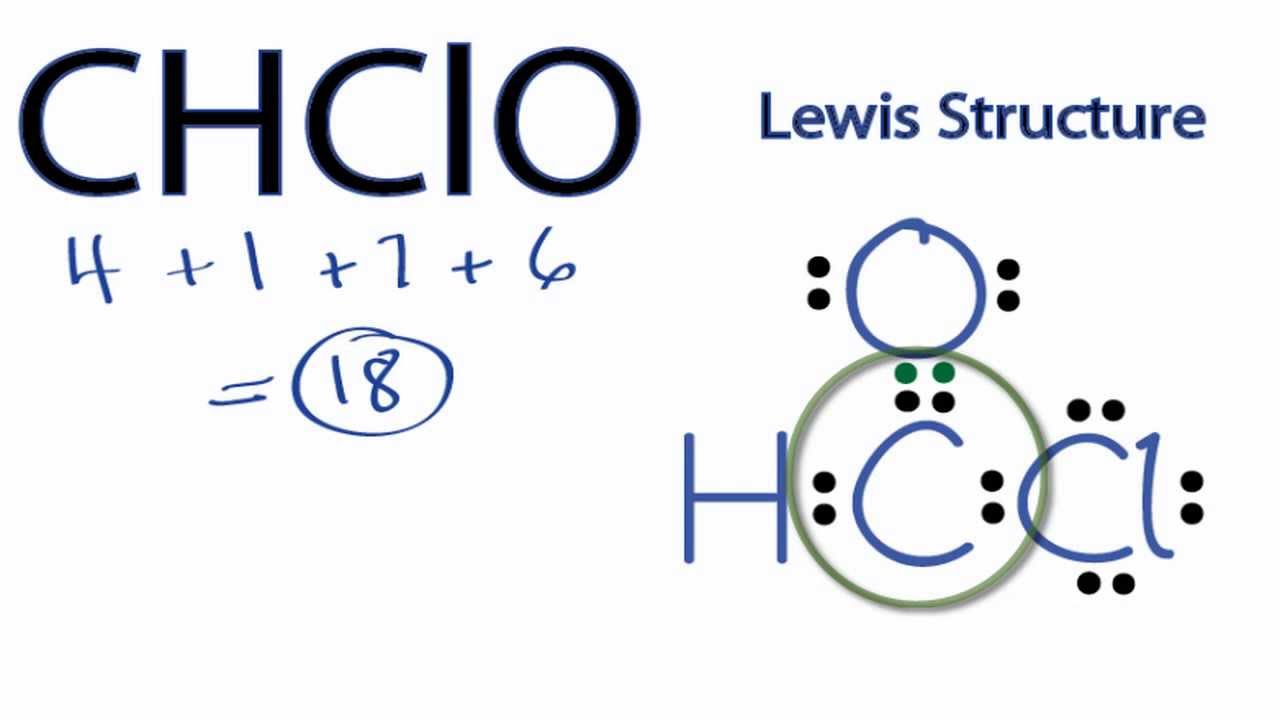
CHClO Lewis Structure How to Draw the Lewis Structure for CHClO YouTube
Determine The Total Number Of Valence Electrons:
Answer Link Given O=C (Cl)H It Is Trigonal Planar To A First Approx.
Draw The Lewis Structure Of C2F6.
Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
Related Post: