Draw The Electron Configuration For A Neutral Atom Of Scandium
Draw The Electron Configuration For A Neutral Atom Of Scandium - Web the electron configuration of a neutral atom in the ground state is 1s22s22p63s2. Web element configuration for atoms or molecules is defined by the number of electrons present in the orbit or shell. The shorthand electron configuration (or noble gas configuration) as. Write the electron configuration for a neutral atom of the element scandium. The atomic number of scandium is 21. Web draw the electron configuration for a neutral atom of scandium. Alternatively, it can be represented as [ne] 3s2 3p6 4s2. Web the atomic symbol of scandium is sc. You'll get a detailed solution from a subject matter expert that helps you learn. Since 1s orbital can hold a maximum of 2. You'll get a detailed solution from a subject matter expert that helps you learn. The electron configuration of a neutral atom of scandium is 1s2 2s2 2p6 3s2 3p6 4s2 3d1. Web in order to write the sc electron configuration we first need to know the number of electrons for the sc atom (there are 21 electrons). The atomic number. Web the atomic symbol of scandium is sc. Write the electron configuration for a neutral atom of the element scandium. Web draw the electron configuration for a neutral atom of sodium. Since 1s orbital can hold a maximum of 2. The [ar] represents the electron configuration of the noble gas argon,. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. The [ar] represents the electron configuration of the noble gas argon,. You'll get a detailed solution from a subject matter expert that helps you learn. Web electron affinity the energy released when an electron is added to. Atomic number of scandium is 21, that is scandium has a total of 21 electrons. Make sure to label each sublevel and. The electron configuration of a neutral atom of scandium is 1s2 2s2 2p6 3s2 3p6 4s2 3d1. (a) draw and orbital diagram for this element. Write the electron configuration for a neutral atom of the element scandium. Olo ar this problem has been solved! Web draw the electron configuration for a neutral atom of sodium. Web in order to write the sc electron configuration we first need to know the number of electrons for the sc atom (there are 21 electrons). The shorthand electron configuration (or noble gas configuration) as. You'll get a detailed solution from a. Web draw the electron configuration for a neutral atom of scandium. You'll get a detailed solution from a subject matter expert that helps you learn. This problem has been solved! Web element configuration for atoms or molecules is defined by the number of electrons present in the orbit or shell. Draw the electron configuration for a neutral atom of iron. In case of scandium, there are 3 orbits and 17. Web draw the electron configuration for a neutral atom of scandium. Web draw the electron configuration for a neutral atom of sodium. You'll get a detailed solution from a subject matter expert that. Electron configuration chart of all elements is mentioned in the table below. Web the electron configuration of a neutral atom in the ground state is 1s22s22p63s2. Web electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. Web scandium’s electron configuration, which is [ar] 3d 1 4s 2, shows that it has one electron in the 3d orbital and two electrons in. Web draw the electron configuration for a neutral atom of scandium. You'll get a detailed solution from a subject matter expert that. Since 1s orbital can hold a maximum of 2. Draw the electron configuration for a neutral atom of scandium. Make sure to label each sublevel and. Write a possible set of quantum numbers for the fourth electron added to the atom, and identify. The [ar] represents the electron configuration of the noble gas argon,. The atomic number of an element is equal to the number of electrons present in a neutral atom. The atomic number tells you how many electrons to draw in total. The shorthand. Olo ar this problem has been solved! Web the electron configuration notation for scandium can be represented as [ar] 4s^2 3d^1. Web draw the electron configuration for a neutral atom of scandium. The atomic number tells you how many electrons to draw in total. Make sure to label each sublevel and. Draw the electron configuration for a neutral atom of iron. Atomic number of scandium is 21, that is scandium has a total of 21 electrons. The atomic number of scandium is 21. The shorthand electron configuration (or noble gas configuration) as. Web gm deco go = o electronic structure drawing a box diagram of the electron configuration of an atom daw the electron configuration for a neutral atom of. Scandium is a transition metal with an. The atomic number of an element is equal to the number of electrons present in a neutral atom. Electronegativity (pauling scale) the tendency of an atom to. Web in this section, we will explore the electron configuration of a neutral atom of scandium (sc) and understand its significance in chemistry. (a) draw and orbital diagram for this element. The electron configuration of a neutral atom of scandium is 1s2 2s2 2p6 3s2 3p6 4s2 3d1.
Scandium electronic configuration How to Write Scandium electronic

Scandium, atomic structure Stock Image C018/3702 Science Photo
:max_bytes(150000):strip_icc()/Scandium-58b6023e3df78cdcd83d49e1.jpg)
Atoms Diagrams Electron Configurations of Elements
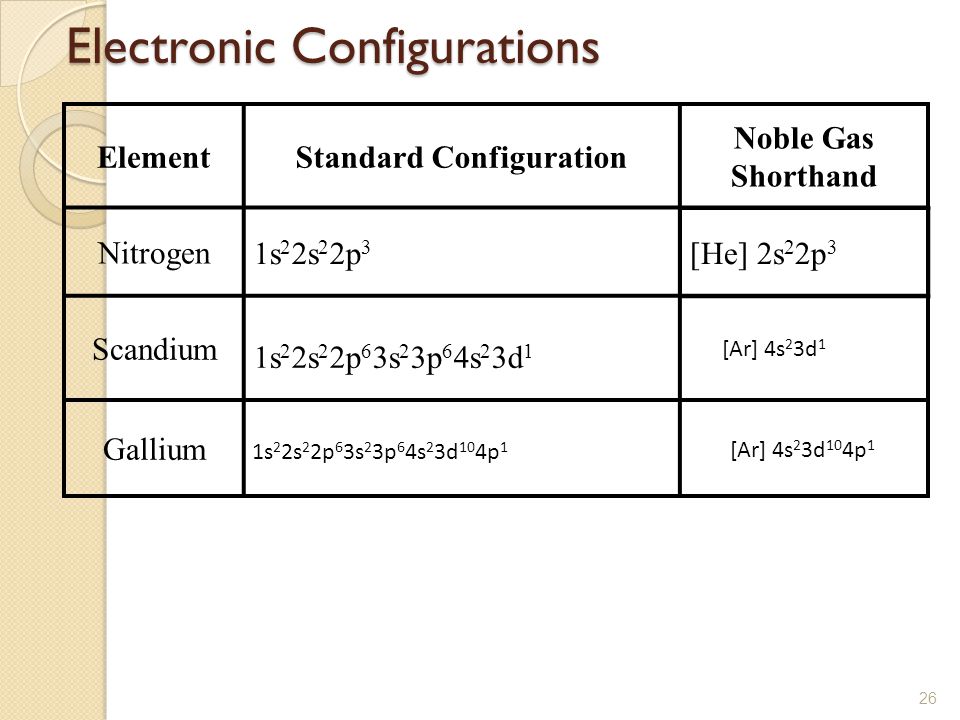
Scandium Electron Configuration (Sc) with Orbital Diagram

Orbital Diagram For Scandium
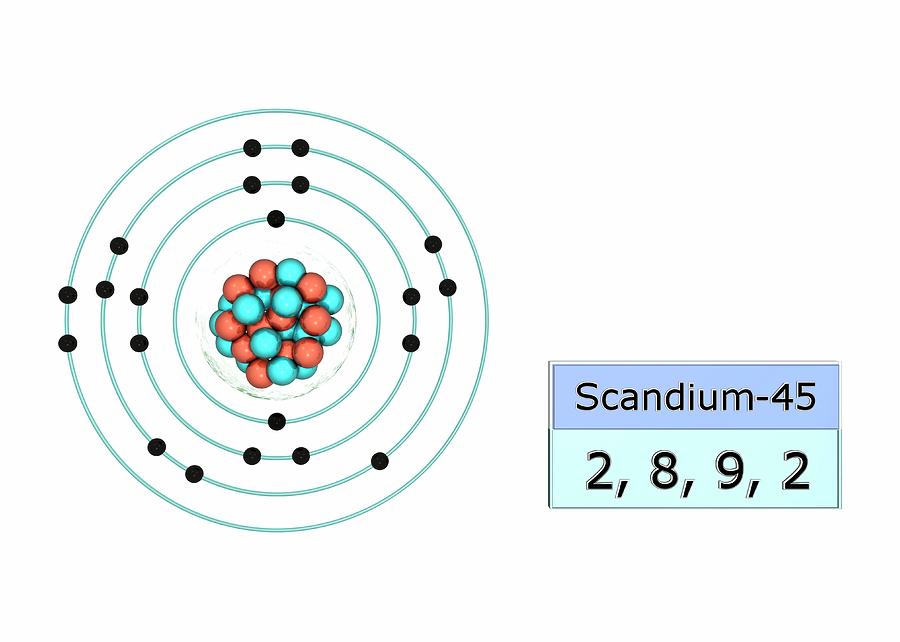
Scandium Electron Configuration Photograph by
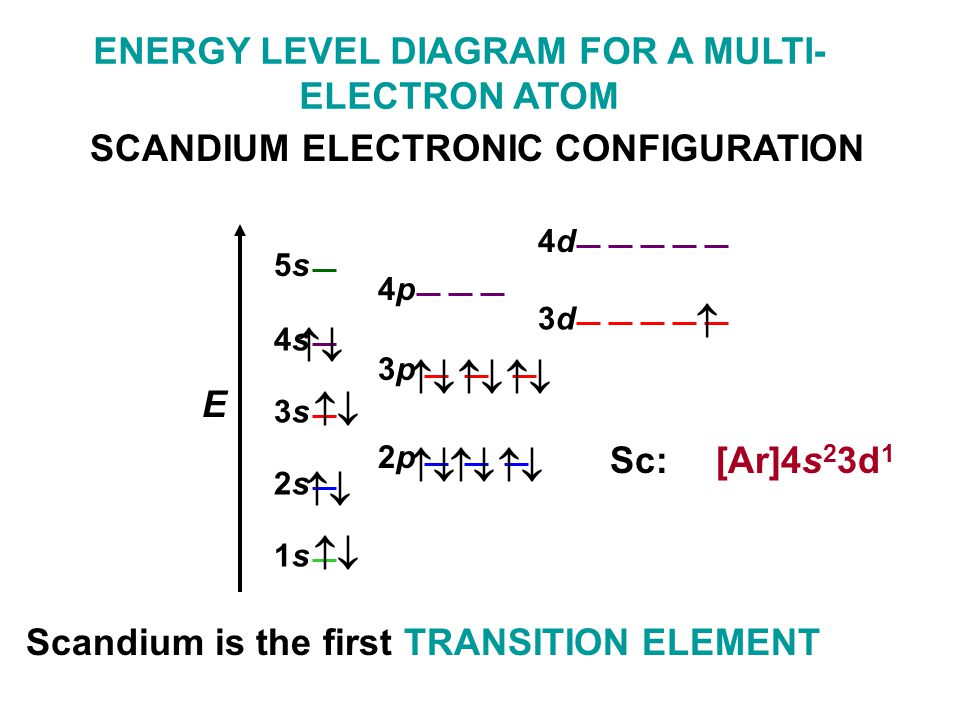
Scandium Electron Configuration (Sc) with Orbital Diagram
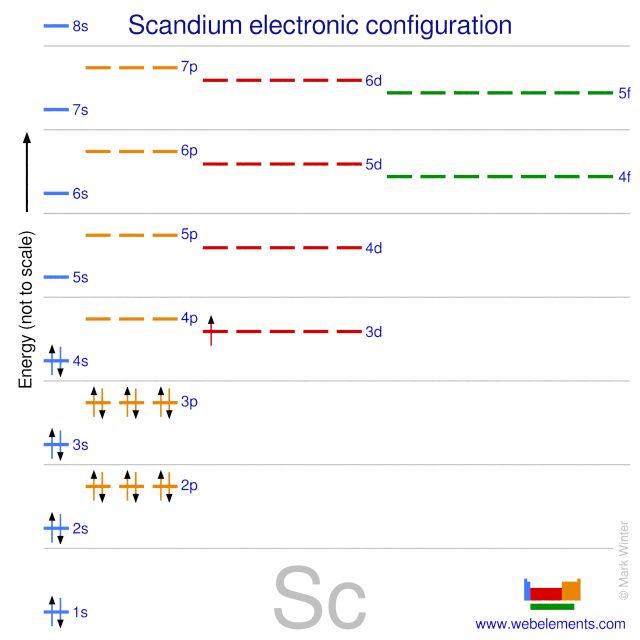
WebElements Periodic Table » Scandium » properties of free atoms

Orbital Diagram For Scandium
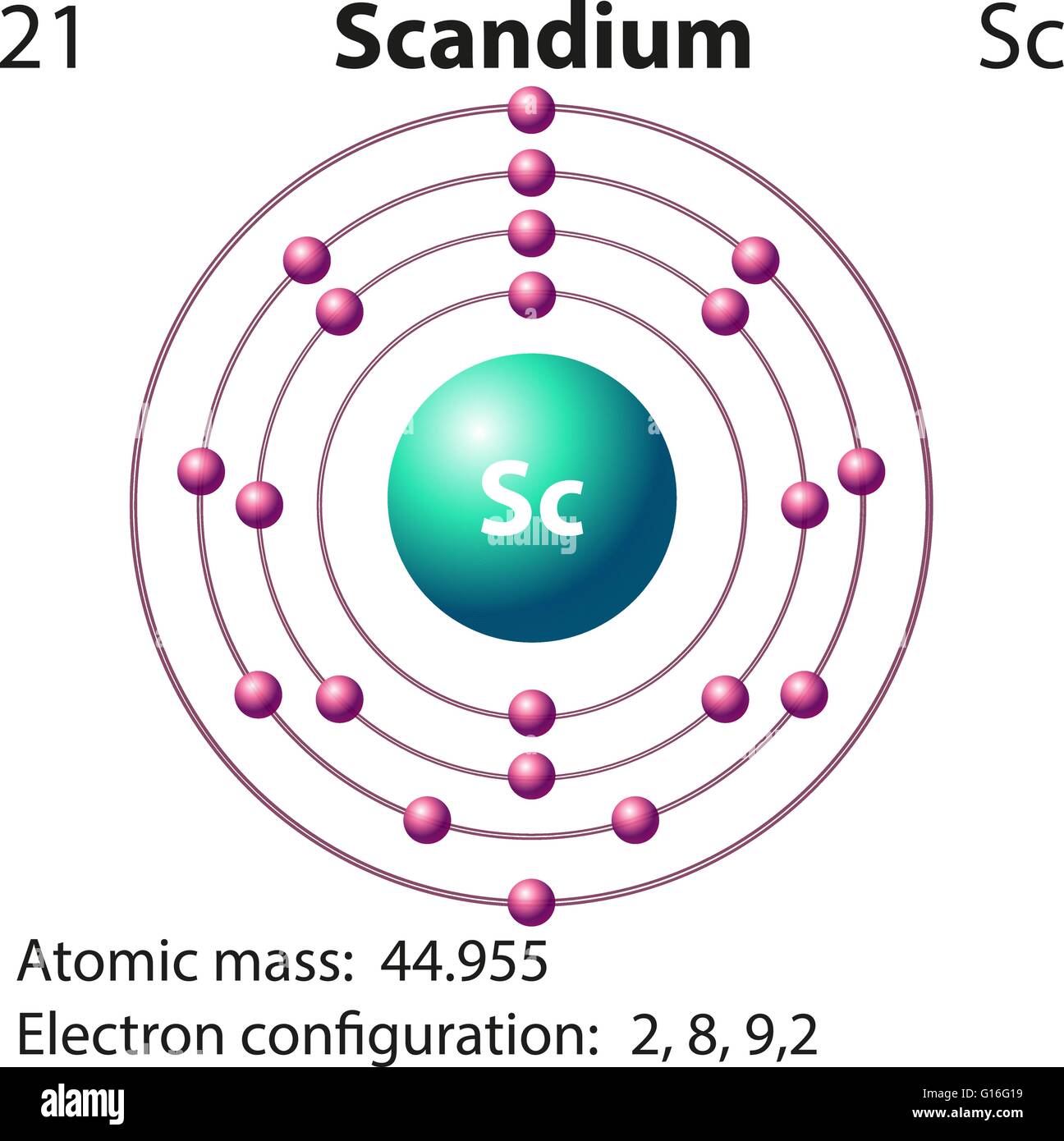
Symbol and electron diagram for Scandium illustration Stock Vector
Draw The Electron Configuration For A Neutral Atom Of Scandium.
Web In Order To Write The Sc Electron Configuration We First Need To Know The Number Of Electrons For The Sc Atom (There Are 21 Electrons).
Find The Element On The Periodic Table.
Web Element Configuration For Atoms Or Molecules Is Defined By The Number Of Electrons Present In The Orbit Or Shell.
Related Post: